C6H12, (E)3Methyl2pentene, Molecules Containing Five or More Carbon Atoms DOI /_447 Study of the composition of gas from the catalytic cracking of vacuum gas oil, by means of capillary gas chromatography, Chemistry and Technology of Fuels and OilsIf the substance is covered by more than one CLH entry (eg disodium tetraborate EC no 215–540–4, is covered by three harmonisations:3Methylpent2ene (CH3CH=C(CH3)CH2CH3) reacts with Hydrogen Chloride(HCl) forming a major and minor product Please name the reaction, draw the mechanism for the formation of the major product and briefly explain why there is a major and a minor product
E 3 Isopropyl 2 Methyl Pent 2 Ene 1 5 Diol C9h18o2 Chemspider
(e)-3-methylpent-2-ene
(e)-3-methylpent-2-ene-Find 3methylpent2ene and related products for scientific research at MilliporeSigma4Methyl3ethyltrans2pentene Permanent link for this species




3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Pero Innovayz
Other names 2Pentene, 3ethyl4methyl, (E);(Z)3Methyl2penten1ol C6H12O CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more603 (estimated with error 39) NIST Spectra mainlib_1144, replib_490, replib_193, replib_ Predicted data is generated using the ACD/Labs Percepta Platform PhysChem Module Density 07±01 g/cm 3 Boiling Point 646±70 °C at 760 mmHg
(E)2Chloro3methylpent2ene C6H11Cl CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and moreA reaction of an unknown alkene with MCPBA in dichloromethane followed by workup with H2O/H yielded, as the major product, a racemic mixture of (2S,3S) and (2R,3R)3 methylpentan2,3diol What is the specific structure of the alkene used in the reaction?005–011–01–1 and 005–011–02–9), CLH information cannot be displayed in the InfoCard as the difference between the CLH classifications requires manual interpretation or
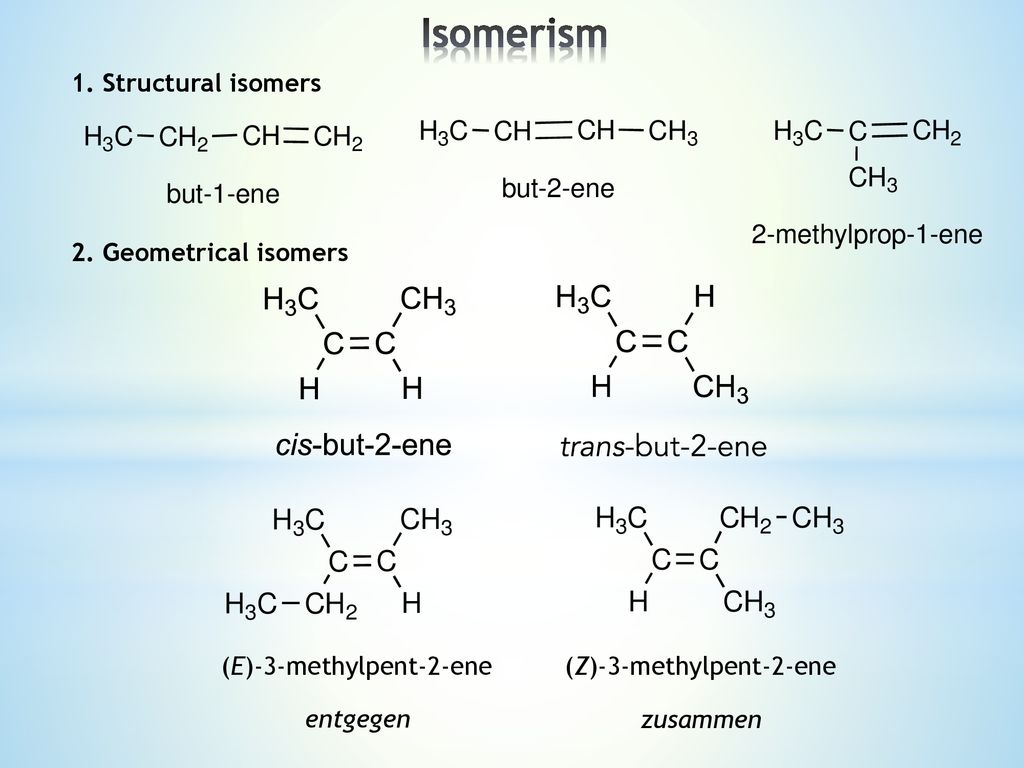
3Methyl2pentene Molecular Formula C 6 H 12 Average mass Da Monoisotopic mass Da ChemSpider ID This record has not been tagged Chemsrc provides 1chloro3methylpent2ene(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of 1chloro3methylpent2ene are included as well#1 Report Thread starter 7 years ago #1 Hi, I'm working on an E/Z isomer question (again) and was wondering if 3methylpent2ene had E and Z isomers?



2e 1 Bromo 3 Methylpent 2 Ene Get Quote



Q Tbn And9gcq Wpcfo1kzqmnlvswhw9sdnhbdkb5npevfx3o9edv2s8wzwrri Usqp Cau
Methylpent2ene C Z3ethylbut2ene D ethylbut2ene A What is the effect produced by the PRK technique designed to correct nearsightedness?Lower right example methylpent2ene and Z3methylpent2ene (repeated further down with skeletal formulae) To understand the two lower left and right examples apply the Priority Rules to alkenes for E/Z ('geometrical') isomerism For each carbon of the double bond the higher priority atom/group is worked outBioaccumulation Estimates from Log Kow (BCFWIN v217) Log BCF from regressionbased method = 1610 (BCF = 407) log Kow used 300 (estimated) Volatilization from Water Henry LC 0423 atmm3/mole (estimated by Bond SAR Method) HalfLife from Model River hours (5624 min) HalfLife from Model Lake 8715 hours (3631 days) Removal In



How Many Different Alkenes Can Be Hydrogenated To Form 3 Methylpentane Socratic



2z 3 Methylpent 2 Ene Get Quote
Structure, properties, spectra, suppliers and links for 2Methylpent2ene,With butan2one(E)1chloro3methylpent2 to form (E)3,6dimethyloct5en2one;File (E)3methylpent2ene 0svg Size of this PNG preview of this SVG file 162 × 56 pixels Other resolutions 3 × 111 pixels 640 × 221 pixels 800 × 277 pixels 1,024 × 354 pixels 1,280 × 442 pixels 2,560 × 5 pixels




13 0 Alkenes Exam Q S Flashcards Quizlet




How To Draw The Structure For 3 Methylpent 1 Ene Drawing Alkenes Organic Chemistry Youtube
With cyclohexanone(E)1chloro3methylpent2 to form 2(Z)3methylpent2enyl(a) The alkene 3methylpent2ene (CH 3 CH=C(CH 3)CH 2 CH 3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene (1) (b) Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromide Explain why more 3bromo3methylpentane is formed in thisChemical structure This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Stereoisomers 3Ethyl4methyl2pentene (Z)3Ethyl4methylpent2ene;
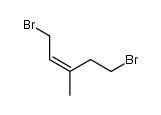



Z 1 5 Dibromo 3 Methylpent 2 Ene Cas 67 2 Chemsrc
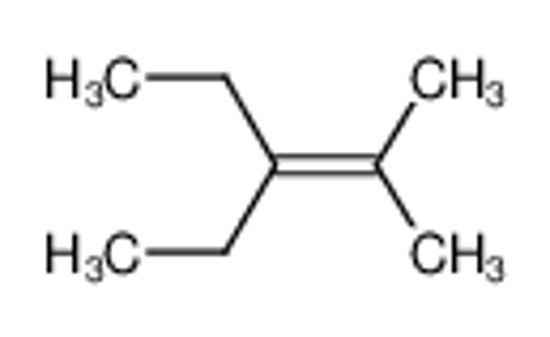



China Low Price 3 Ethyl 2 Methylpent 2 Ene Manufacturers Suppliers Factory Direct Wholesale Globalchemmall
(E)3Methylpent2en Chemische Eigenschaften,Einsatz,Produktion Methoden RSätze Betriebsanweisung R11Leichtentzündlich R65Gesundheitsschädlich kann beim Verschlucken Lungenschäden verursachen SSätze Betriebsanweisung S9Behälter an einem gut gelüfteten Ort aufbewahren S16Von Zündquellen fernhalten Nicht rauchenComputed by Cactvs (PubChem release ) Rotatable Bond Count 1 Computed by Cactvs (PubChem release ) Exact Mass Computed by PubChem 21 (PubChem release ) Monoisotopic Mass Computed by PubChem 21 (PubChem release ) Topological Polar Surface Area 0 ŲQuestion Select The IUPAC Name For The Compound Shown Below 0 (E)2methylpent3ene (E)3methylpent2ene (E)3methylpent3ene O (Z)3methylpent2ene



3 Ethyl 2 Methylpent 2 Ene Get Quote



E 3 Methylpent 2 Ene Molbase
4 Briefly explain how to assign priority to groups using CIP nomenclature a Use CHClC(CH 3)CH 2 CH 3 to demonstrateEnglish Structure of (Z)3methylpent2ene Deutsch Struktur von (Z)3Methyl2penten Date 12 March 14 Source Own work Author Emeldir SVG development The source code of this SVG is valid This structural formula was created with Name2Struct CS ChemDraw UltraInChI=1/C6H11Cl/c146 (7)5 (2)3/h45H,13H3/b64 Molecular Formula C6H11Cl Reactions where this compound is a product ( Cross Metathesis) 0 from prop1ene, 2chloro3methylbut1ene from 2chloro3methylbut1ene, prop1ene Reactions where this compound is




3 Methyl 3 Penten 2 One 98 Acros Organics Alpha Beta Unsaturated Carbonyl Compounds Carbonyl Compounds Fisher Scientific
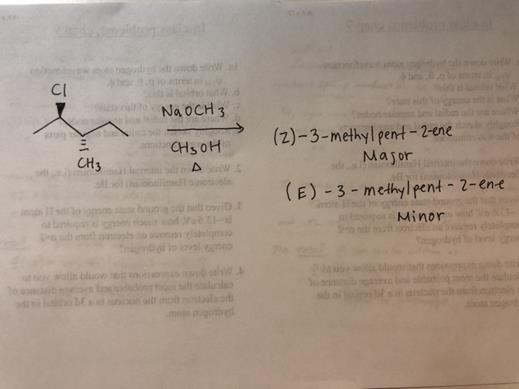



Get Answer 2r 3s 2 Chloro 3 Methylpentane Reacts With Sodium Methoxide In Transtutors
A The density of the cornea is increased B The radius of curvature of the cornea is increased CThis structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Stereoisomers 3Ethyl4methyl2pentene (Z)3Ethyl4methylpent2ene Other names 2Pentene, 3ethyl4methyl, (E);The compound is (E)but2ene A minor addition to the rule to allow for isotopes of, for example, hydrogen Deuterium is an isotope of hydrogen having a relative atomic mass of 2 It still has only 1 proton, and so still has an atomic number of 1 However, it isn't the same as an atom of "ordinary" hydrogen, and so these two compounds are



Answer In Organic Chemistry For Edem



1
Chemical structure of (2E)3methylpent2ene See it's properties and synonyms(a)€€€€ The alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene (1) 4 (b)€€€€ Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromideNIST/TRC Web Thermo Tables (WTT) NIST Standard Reference Subscription Database 3 Professional Edition Version 2121Pro This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics These data were generated through dynamic data analysis, as implemented in the NIST



9 19 Additional Exercises Chemistry Libretexts



E 1 4 Dichloro 3 Methyl Pent 2 Ene Chemsink
Explain why 3methylpent2ene does not display cis trans isomerism, but does display E/Z isomerism c Does 2methylpent2ene satisfy any of the criteria?2Pentene, 3methyl, (E) Formula C 6 H 12 Molecular weight IUPAC Standard InChI InChI=1S/C6H12/c146 (3)52/h4H,5H2,13H3/b64 Copy Sheet of paper on top of another sheet IUPAC Standard InChIKey BEQGRRJLJLVQAQGQCTYLIASAN Copy Sheet of paper on top of another sheetA) (Z)3methylpent2ene B) (E)3methylpent 2ene C) 2methylpent2ene
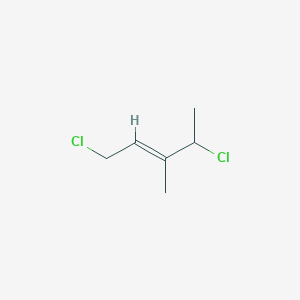



E 1 4 Dichloro 3 Methylpent 2 Ene C6h10cl2 Pubchem



E 3 Ethyl 4 Methylpent 2 Ene
Chemsrc provides cis3Methyl2pentene(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of cis3Methyl2pentene are included as wellQ select the incorrect statement answer choices In alkenes, the carbons are connected by pi bonds Alkenes have almost same physical properties as that of the alkanes Alkenes are less reactive than alkanes Alkenes undergo polymerization reactions s Question 3Find SigmaAldrich MSDS, related peerreviewed papers, technical documents, similar products & more at SigmaAldrich



Z 3 Ethyl 4 Methylpent 2 Ene




Z 3 Methylpent 2 En 1 Ol Structure C6h12o Over 100 Million Chemical Compounds Mol Instincts
InChI=1/C6H11Cl/c145 (2)6 (3)7/h4H2,13H3/b65 Molecular Formula C6H11Cl Reactions where this compound is a product ( Cross Metathesis) 0 from 2methylbut1ene, 2chloroprop1ene from 2chloroprop1ene, 2methylbut1ene More Info at PubChem The structure of (E)3methylpent2ene is According to Markovnikov's rule, the H from HBr will add to C2 of the alkene and form a 3° carbocation at C3 The Br− will add to C3 and form 3bromo3methylpentane There may be a small amount of product formed when the H adds to C3 of the alkene to form the less stable 2° carbocation at C2CAS Registry Number ;




3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Pero Innovayz



E 2 Chloro 3 Methyl Pent 2 Ene Chemsink
3methylpent2ene isomer Watch start new discussion reply Page 1 of 1 Go to first unread Skip to page Veqz Badges 4 Rep?CAS Registry Number Chemical structure This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Stereoisomers 3Ethyl4methyl2pentene (Z)3Ethyl4methylpent2ene Other names 2Pentene, 3ethyl4methyl, (E);The (E)1chloro3methylpent2ene molecule contains a total of 17 bond (s) There are 6 nonH bond (s), 1 multiple bond (s), 1 rotatable bond (s) and 1 double bond (s) Images of the chemical structure of (E)1chloro3methylpent2ene are given below 2dimensional (2D) chemical structure image of (E)1chloro3methylpent2ene




Organic Chemistry Alkenes



Secondaryscience4all Files Wordpress Com 14 05 Mini Mock As Amount Of Substance Bonding Analytical Techniques Mon 28 Apr 14 Pdf
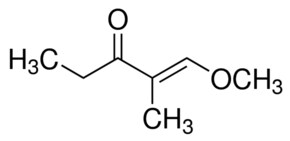



1e 1 Methoxy 2 Methyl 1 Penten 3 One 35 7




Write Structural Formulas And Give The Iupac Names Full Size Png Download Seekpng
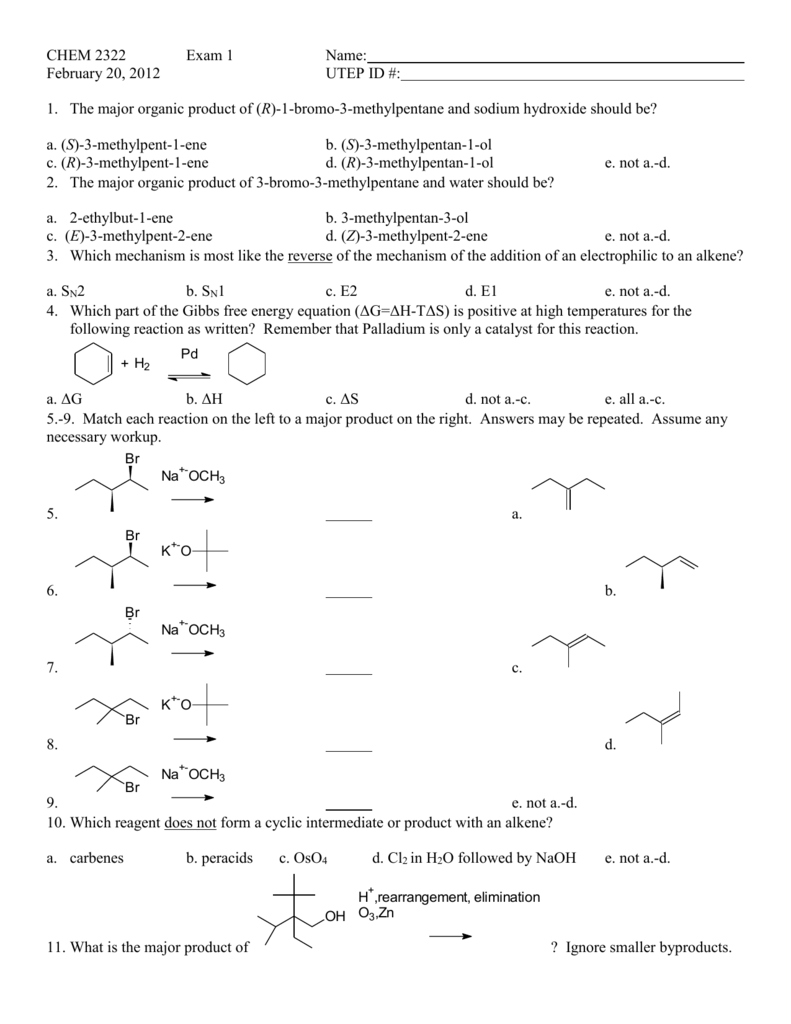



Spring 12 Chem 2322 Exam 1
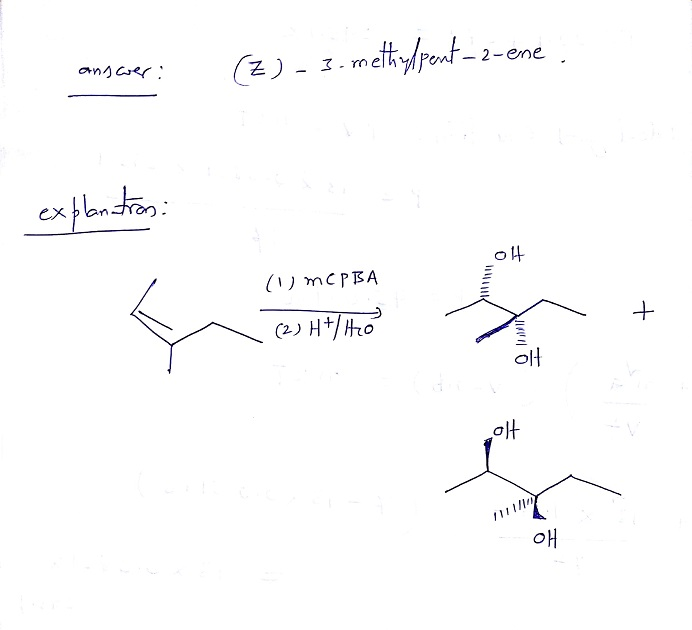



A Reaction Of An Unknown Alkene With Mcpba In Dichloromethane Followed By Work Up With Answersbay




3 Methylpent 2 Ene Sigma Aldrich




3 Chloro 2 Methylpent 2 Ene 71 6 Wiki
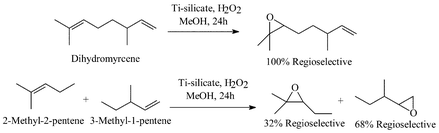



Shape Selective Oxidation Using Titanium Silicates Epoxidation Of Dihydromyrcene And The Model Compounds 2 Methylpent 2 Ene And 3 Methylpent 1 Ene Journal Of The Chemical Society Perkin Transactions 2 Rsc Publishing



E 3 Isopropyl 2 Methyl Pent 2 Ene 1 5 Diol C9h18o2 Chemspider
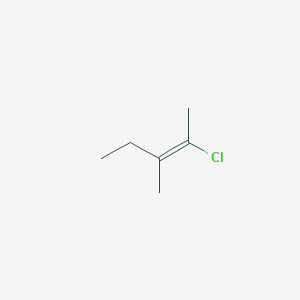



E 2 Chloro 3 Methylpent 2 Ene C6h11cl Pubchem
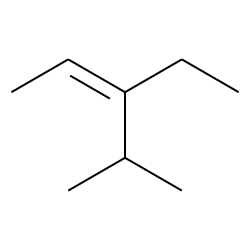



Z 3 Ethyl 4 Methylpent 2 Ene Cas 467 48 1 Chemical Physical Properties By Chemeo



Z 3 Methylpent 2 Ene Chemsink




Which Of The Following Is The Major Product In The Electrophilic Addition Of Hcl To 2 Methylpent 2 Ene Homeworklib




Trans 3 Methylpent 2 Ene Is




Organic Chemistry Alkenes
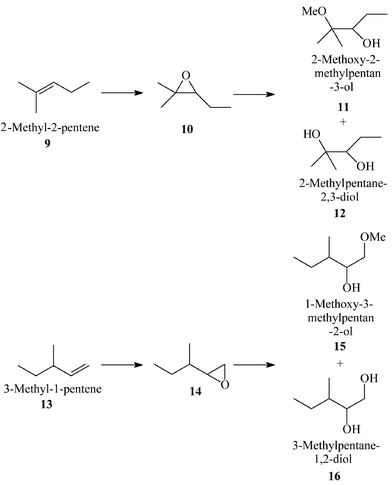



Shape Selective Oxidation Using Titanium Silicates Epoxidation Of Dihydromyrcene And The Model Compounds 2 Methylpent 2 Ene And 3 Methylpent 1 Ene Journal Of The Chemical Society Perkin Transactions 2 Rsc Publishing Doi 10 1039 425g
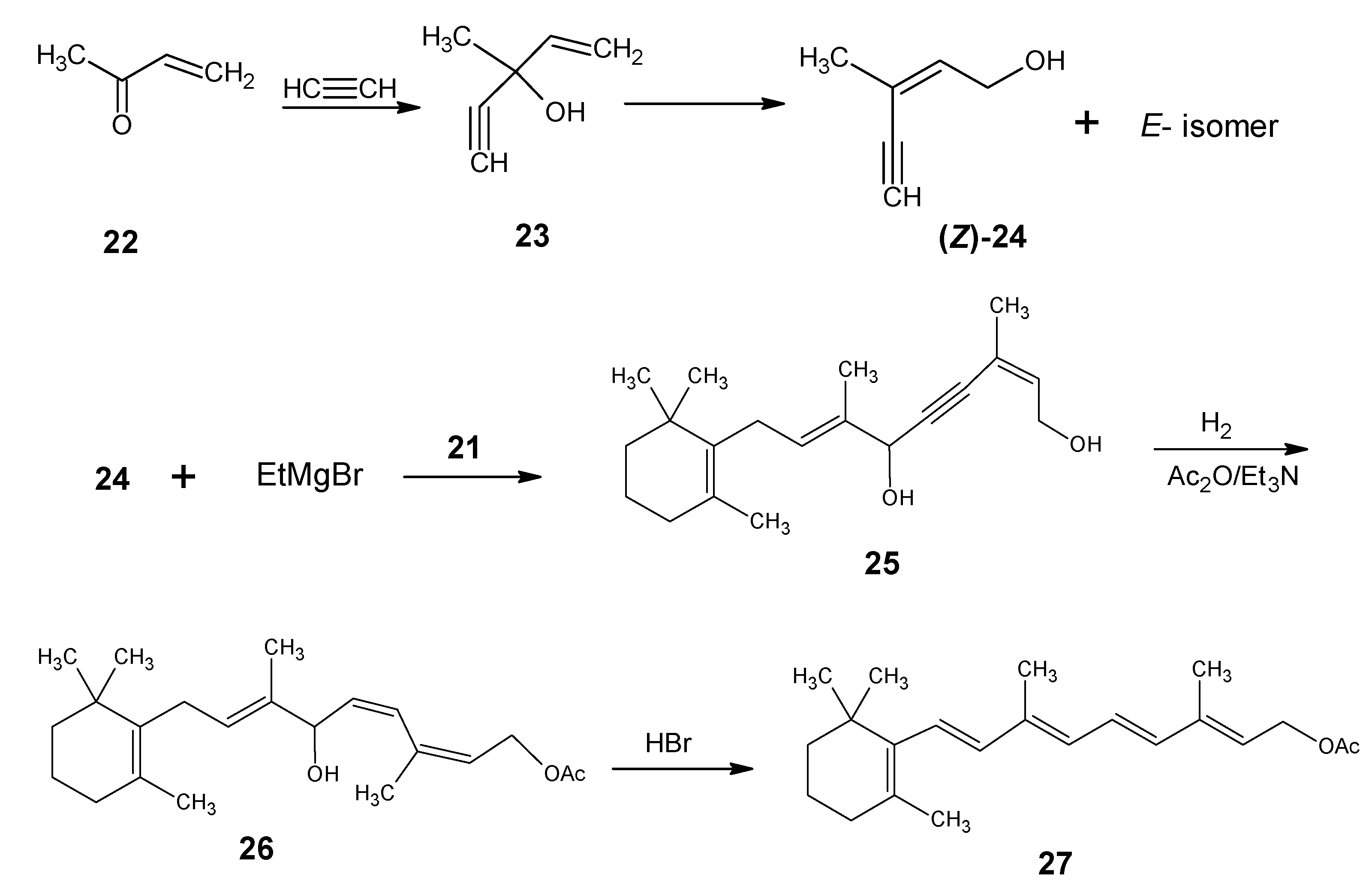



Molecules Free Full Text Synthesis And Use Of Stable Isotope Enriched Retinals In The Field Of Vitamin A Html




Write Down The Correct Mechanism For The Reaction Between 3 Methyl 2 Pentene And Hcl Study Com




3 Methyl 2 Pentene Cis And Trans Mixture 99 0 Tci America Fisher Scientific




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons
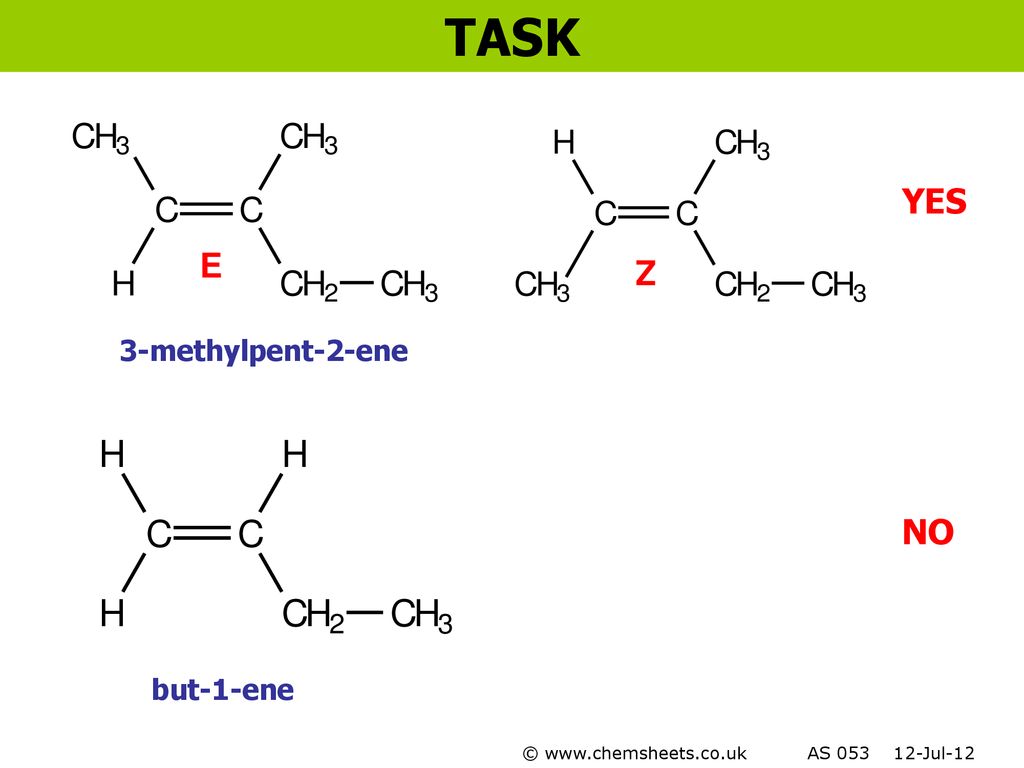



Chemsheets As006 Electron Arrangement Ppt Download



Solved What Is The Major Product When 3 Methylpent 2 Ene Reacts With 9 n Then H2o2 Oh H2o Course Hero




13 0 Alkenes Exam Q S Flashcards Quizlet




Stereoisomerism Wikiwand



Cas 6153 06 6 Products Price Suppliers




Geometrical Isomerism Cis Trans In Trans 2 Fluoro 3 Methylpent 2 Ene Chemistry Stack Exchange



2 Ethyl 3 Methyl 1 Pentene




When 3 Methylpent 2 Ene Is Treated With Mercury Ii Acetate In Methanol And The Resulting Product Brainly Com



E 3 Methylpent 2 Enenitrile Chemsink
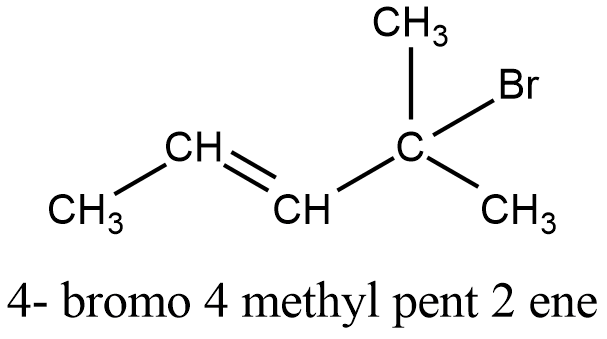



Write The Structure Of Four Bromo 4 Methyl Pent 2 Ene Chemistry Topperlearning Com N3bifjj
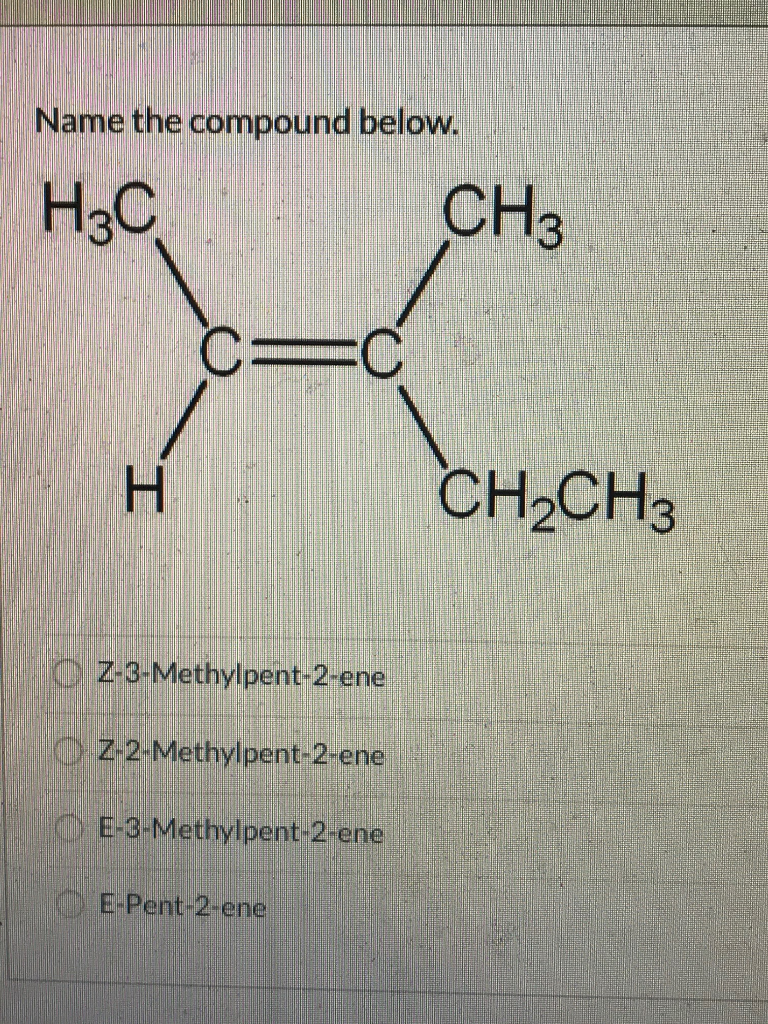



Name The Compound Below H3c Ch3 C C H Ch2ch3 Chegg Com



2747 48 0 2e 3 Methylpent 2 En 1 Ol Cas No 2747 48 0 2e 3 Methylpent 2 En 1 Ol




3 Methylpent 2 Ene On Reaction With Hbr In Presence Of Perox



Alkenes Lecture Ppt Download




2e 2 Chloro 3 Methylpent 2 Ene Structure C6h11cl Over 100 Million Chemical Compounds Mol Instincts



Which Of The Following Compounds Are Capable Of Ciis And Trans Isomerism Why 4 Methylpent 2 Ene 3 Methylpent 1 Ene 4 Chlorohex 2 Ene Quora



E 3 Methylpent 2 Ene Molbase




E 2 3 Dibromo 4 Methylpent 2 Enoic Acid
-2-bromo-3-methylbutane_small.png)



Organic Chemistry Alkenes
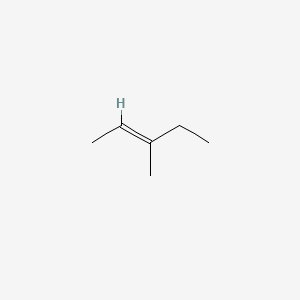



Trans 3 Methyl 2 Pentene C6h12 Pubchem



4461 48 7 4 Methylpent 2 Ene Cas No 4461 48 7 4 Methylpent 2 Ene




Draw The Bond Line Structures Of The Following Compound Whose Iupac Names Are Given As Under A 2 Bromobutane B 1 Chloro 3 Methylbutane C 2 Bromo 2 Methylpropane D 4 Chloro 4 Methylpent 2 Ene E




E 2 Ethoxy 3 Methylpent 2 Ene Structure C8h16o Over 100 Million Chemical Compounds Mol Instincts




Chemsheets As006 Electron Arrangement Ppt Video Online Download



1
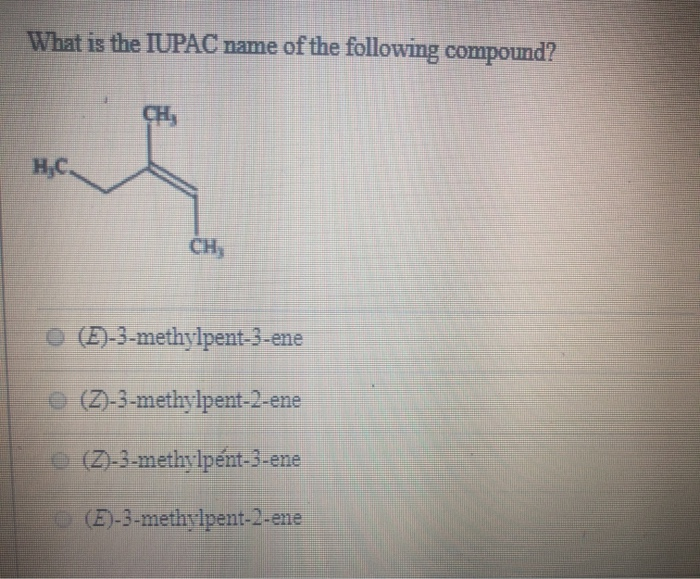



What Is The Iupac Name Of The Following Compound Eh Chegg Com




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons




Solve It 3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Peroxide Forms An Addition Product The Number Of Possible Stereoisomers For The Product Is
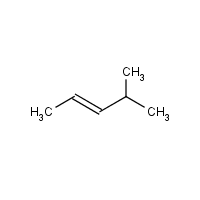



E 4 Methylpent 2 Ene Hazardous Agents Haz Map



Scilearn Sydney Edu Au Fychemistry Textbook Chapter15 Pdf




Draw The Structure S Of The Alkene S With The Molecular Formula C6h12 That Have A Single Methyl Homeworklib




3 Methyl 3 Penten 2 One Wikipedia



Answer In Organic Chemistry For Michael




E 3 Methyl 4 Oxopent 2 Enenitrile Spectrabase




3 Methylpent 2 Ene 1 5 Diol Chemical Physical Properties By Chemeo



Does 3 Methyl 2 Pentene Have Cis Trans Isomers Quora




Help Mcat
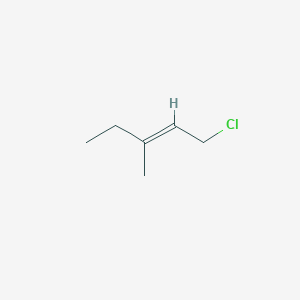



E 1 Chloro 3 Methylpent 2 Ene C6h11cl Pubchem




2e 4 Bromo 3 Methyl 2 Pentenoic Acid C6h9bro2 Density Melting Point Boiling Point Structural Formula Synthesis



Echa Europa Eu Substance Information Substanceinfo 100 010 561
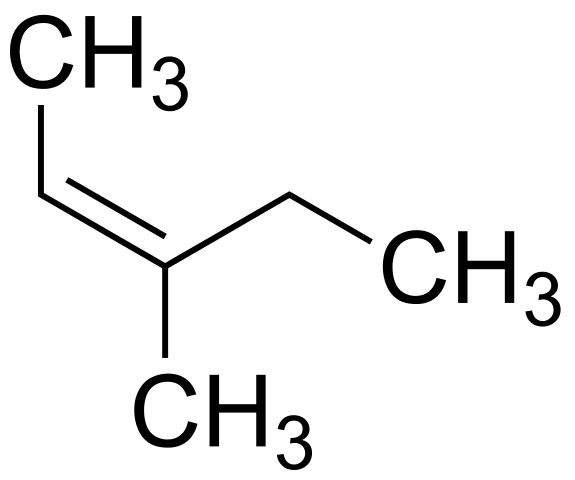



File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons



Cis 3 Methylpent 2 Ene



Solved 1 Draw A 2 Methylpent 1 Ene B Propyne C Non 2 Yne D 4 Methylhex 2 Ene E Methylcyclobutane F 3 Methylcyclooct 1 Yne G But 1 Ene H Course Hero



What Are The Major And Minor Products Obtained In The Reaction Of E 3 Methyl 2 Pentene And Hbr Socratic



Z 3 Ethyl 4 Methylpent 2 Ene C8h16 Chemspider




3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Peroxide Forms An Addition Product The Number Of Possible Stereoisomers For The Products



Echa Europa Eu Substance Information Substanceinfo 100 009 515
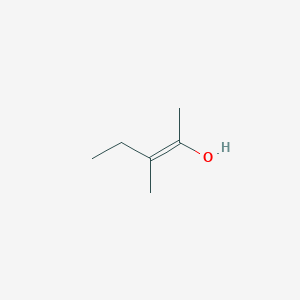



E 3 Methylpent 2 En 2 Ol C6h12o Pubchem




File E 3 Methylpent 2 Ene 0 Svg Wikimedia Commons
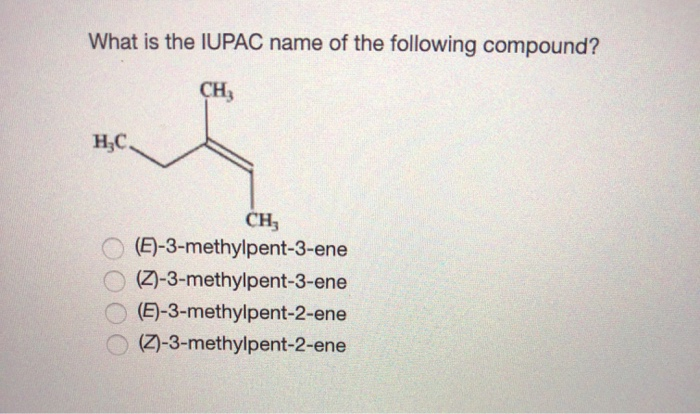



What Is The Iupac Name Of The Following Compound Ch Chegg Com




10 8 Anti Markovnikov Additions To Alkenes And Alkynes Organic Chemistry 1 An Open Textbook
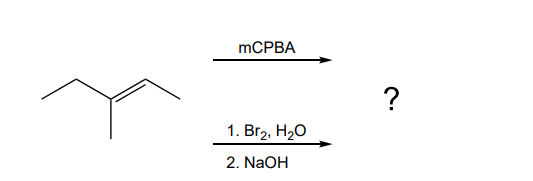



When E 3 Methylpent 2 Ene Is Reacted With Either Chegg Com



2e 3 Methyl 2 Pentene C6h12 Chemspider
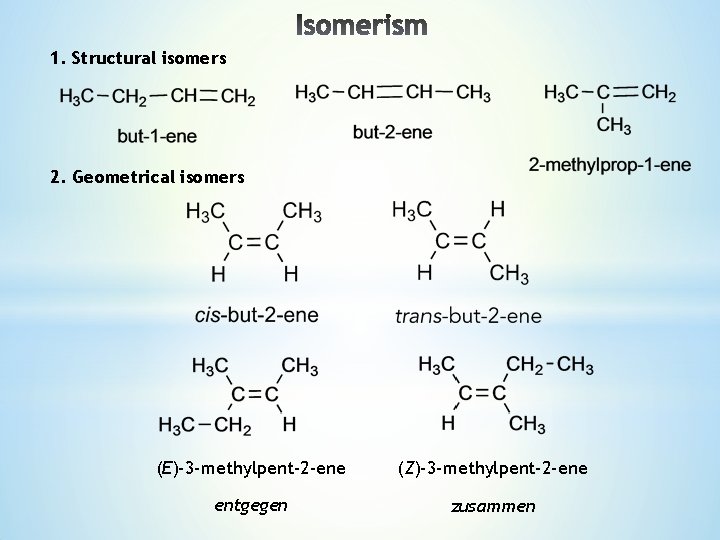



Lecture 4 1 Structural Isomers 2 Geometrical Isomers



3 Methylpent 2 Ene 1 5 Diol C6h12o2 Chemspider



E 3 Methylpent 2 Ene Molbase



1
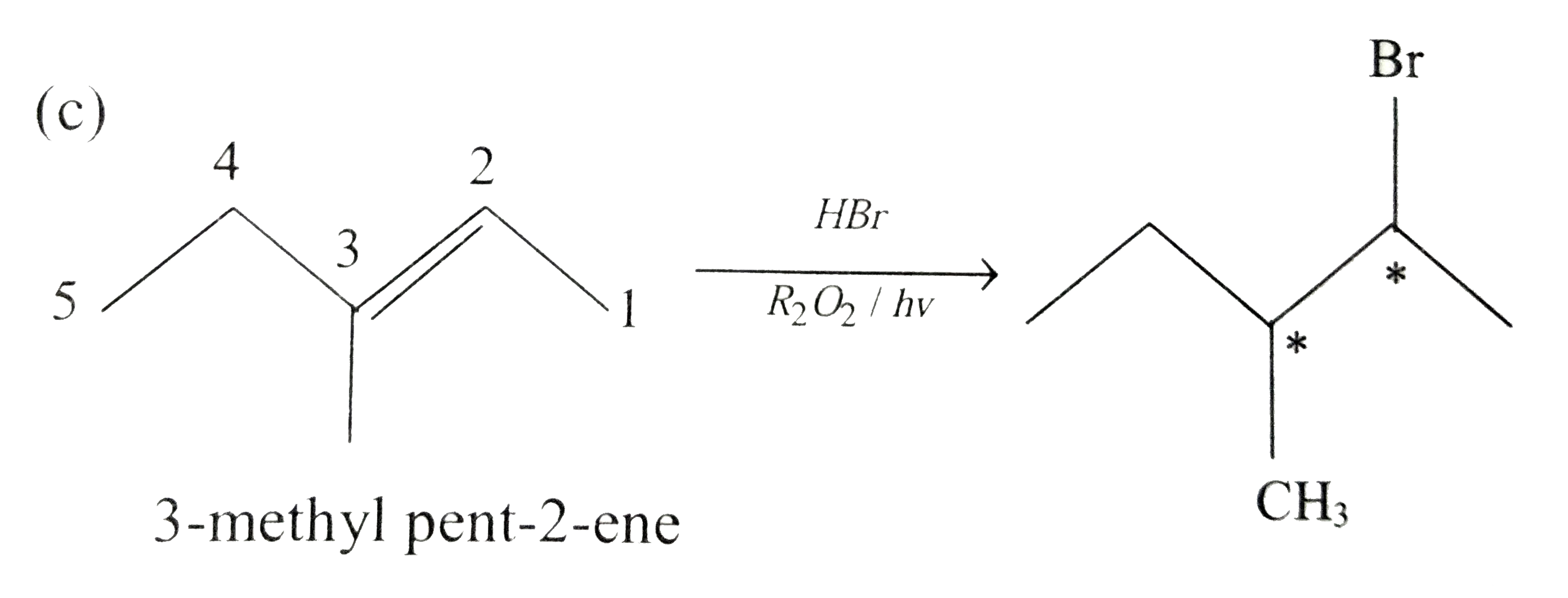



3 Menthyl Pent 2 Ene On Reaction With Hbr In Presence Of Peroxide Forms An Addition Product The Number Of Possible Stereoisomers For The Product Is



2e 3 Methylpent 2 Ene Get Quote




13 0 Alkenes Exam Q S Flashcards Quizlet
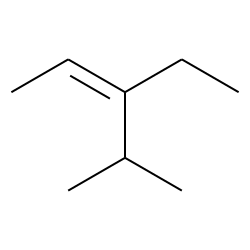



E 3 Ethyl 4 Methylpent 2 Ene Cas 467 49 2 Chemical Physical Properties By Chemeo



0 件のコメント:
コメントを投稿